Dr. Rehm, Board certified in Diabetic Foot Wound Care, practices in San Diego. He lectures nationally and offers seminars for podiatrists and other professionals. Dr. Vayser is on staff in the Division of Orthopaedic Surgery, Scripps Clinic, La Jolla, CA. Dr. Stoddard is in private practice in Massilon, OH. His contribution was submitted during residency at Med Premises Surgical Center, San Diego, CA. Dr. Patel is chief resident at Med Premises Surgical Center, San Diego, CA.
|
After reading this continuing education article, the podiatric physician should be able to: 1. Describe and define osteomyelitis and its variants. 2. Identify and describe in detail the different forms of osteomyelitis found in the diabetic foot. 3. Describe the pathophysiology and clinical course of osteomyelitis in the diabetic foot. 4. Understand the essentials of diagnosis of osteomyelitis in the diabetic foot. 5. Understand the indications and use imaging modalities used in the diagnoses of osteomyelitis in the diabetic foot. 6. Be familiar with the bacteriology and the use of antimicrobial therapy in the treatment of osteomyelitis in the diabetic foot. 7. Understand the treatment protocols and the relationship of conservative medical and surgical treatment vs. amputation in the diabetic patient with osteomyelitis |
Osteomyelitis of the foot in diabetic patients is typically encountered in elderly patients with a long history of diabetes. It is a common result of infected foot ulcerations and complicated by peripheral vascular disease and peripheral neuropathy seen commonly in the podiatrist's office. The disease has significant morbidity. Knowing the predictors, presenting signs and symptoms, diagnosis and treatment of foot bone infection in the diabetic should be of primary concern to every podiatrist who sees diabetic patients.
In the medical community, conservative treatment of foot osteomyelitis in the diabetic patient with vascular disease and/or neuropathy is commonly seen as useless. Amputation therefore becomes the treatment of choice. (1,2) More hospitalizations are due to diabetic foot infections than any other complication of diabetes. According to many experts, it is estimated that between one third and two thirds of all patients who present with foot infections are found to have evidence of osteomyelitis (3, 4, 5) Diabetes is considered to be the major cause of lower extremity amputations in the United States, and foot infections are a major contributing factor. (6,7) The podiatrist, therefore, has an opportunity, because of his or her extensive access to, and knowledge of, the diabetic foot, to render cost-effective preventive measures and intervention before amputation becomes the only alternative.
Definitions
Nelaton (1844) is credited as the first to use the term osteomyelitis to describe infection of bone and marrow. Osteomyelitis is generally referred to as a bacterial infection. However, fungal, viral, and parasitic infections have also been documented. (8) In the diabetic foot, all infections of the foot are loosely called osteomyelitis, but in the strict sense of the word, this is a misnomer. (3) The infectious process may involve only the periosteum which would technically be called "periostitis". If the infection involves only the cortex and has not spread to the medullary canal it would be more appropriately termed "osteitis". In fact, most of the small bones of the foot have very little marrow.
Classification
There are two main classifications systems used to identify osteomyelitis in the diabetic foot. The first was described by Waldvogel and colleagues and categorizes bone infections into those that result from hematogenous spread of infection and that which results from spread to the bone from a contiguous focus. Contiguous infections are subcategorized into those with and without vascular insufficiency. These infections are then further classified into acute or chronic osteomyelitis. (10)

Hematogenous osteomyelitis is much more common in children because of the anatomy of the vasculature surrounding open epiphyses. It also affects the elderly due to degenerative changes in the bony architecture. Clinically, one sees abrupt onset of pain, tenderness and inflammation with elevated ESR, PMN's, and CRP. This is treated medically in children and surgically in adults.
Contiguous osteomyelitis is infection of the bone from surrounding infected tissue. In the diabetic foot, bone infection generally develops in this way. This mode of infection is also common in peripheral vascular disease, rheumatic disease, neoplastic disease and drug abuse with corticosteroids and antimetabolites. After foot surgery, contiguous osteomyelitis happens when toenail surgery is combined with surgery of the bone. Foot surgeries where implants are used poses a higher risk of contiguous osteomyelitis as well. Open fractures pose a threat of imminent or impending osteomyelitis as well. (McGlamry)
Osteomyelitis with peripheral vascular disease is usually associated with gangrene. Poor outcome is expected unless the vascular component is addressed first. Modalities such as pulsed high-frequency electromagnetic therapy and diastolic limb pumps have shown promise in this area as reported by podiatrists using these treatments.
Acute vs. Chronic Osteomyelitis
Acute osteomyelitis is a suppurative infection accompanied by edema, vascular congestion and small vessel thrombosis.
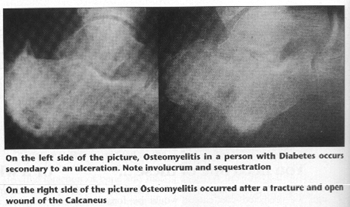
Chronic osteomyelitis is insidious in nature and involves necrotic or ischemic bone, an ischemic soft tissue envelope, or scar tissue. The clinical course is usually refractory. Chronic osteomyelitis is associated with necrotic bone that has become separated from healthy tissue, called sequestrum. New bone formation around this sequestrum is termed involucrum. A localized bone infection may occur where only a small focus of cancellous or cortical bone is affected and is circumscribed by dense fibrous tissue and sclerotic bone. This is called a Brodie Abscess and results either from low virulence of the organism or high resistance of the host.
The second main classification system is recommended by Cierny and Mader (11). This method incorporates anatomic disease types and physiological host factors, resulting in 12 clinical stages of osteomyelitis. The anatomic stages described are: 1) Medullary osteomyelitis 2) Superficial osteomyelitis 3) Localized osteomyelitis 4) Diffuse osteomyelitis. If superficial osteomyelitis is particularly severe or longstanding, it can progress to localized or diffuse osteomyelitis. These are considered in relation to the physiological state of the host: A) Normal Host B) Physiologically compromised host (Locally- BL and/or Systemic BS) C) The host will not be able to tolerate the treatment such that the treatment becomes worse than the disease. In most cases, osteomyelitis in the diabetic foot usually is classified as a 2BSL, that is superficial osteomyelitis in a physiologically compromised host both systemically (diabetes mellitus) and locally (neuropathy and/or vasculopathy).
Pathophysiology
Osteomyelitis in the diabetic patient is largely a consequence of the major complications of diabetes in general. Neuropathy, angiopathy and immunopathy, and delayed wound healing play a major role in the susceptibility and development of infections in the diabetic. This definitely includes bone infection. Neuropathic changes are present in over 80 percent of patients with diabetes related foot disease (12). Neuropathy contributes to osteomyelitis through increased risk of ulceration and soft tissue infection in the foot, mainly in three ways. 1) In diabetic neuropathy, the patient loses protective sensation in the foot. This predisposes the patient to thermal and mechanical injury as well as increased risk of Charcot fracture and deformity 2) Motor neuropathy affecting the extensor and flexor muscles of the leg and foot as well as the intrinsic muscles of the foot causes muscle weakness and contractures. This causes biomechanical imbalance and consequent foot deformities such as hallux abducto valgus and hammertoes, predisposing the patient to foot ulceration and infection. 3) Autonomic neuropathy interferes with sweating and is largely responsible for dry skin and fissures acting as a nidus for skin infection. In addition, diabetic patients develop thicker calluses as well, which causes more pressure to the underlying normal tissues making ulceration more likely. (13)
Even though neuropathy is considered a more important factor, peripheral angiopathy, decreased blood flow through diminished arterial and capillary blood supply, is an important contributor to the development of osteomyelitis through diminished healing capacity and increased necrosis of tissue.
Immunopathy, due to basic immunologic deficiencies in the diabetic patient, makes them more susceptible to various types of infections including bacterial and fungal infections of the skin, and diminishes the wound healing capabilities. Superficial fungal skin infections allow entry of bacteria through open lesions, cracked or macerated skin. In addition, diabetic patients have a high degree of colonization on the skin of the virulent pathogen, staphylococcus aureus, that may take advantage of any decrease in host immunity or defects in the skin.
The diabetic patient is more likely to develop skin ulcerations, has decreased wound healing ability increasing the risk of infection and a decreased ability to fight off that infection. This scenario is the main factor predisposing diabetic patients to osteomyelitis.
Diagnosis
Early accurate diagnosis of osteomyelitis in the diabetic foot is essential and is accentuated by the fact that tissue necrosis may necessitate amputation in as many as 40 percent of patients. (14). Osteomyelitis is a common complication of foot ulcerations in the diabetic, occurring in as many as 15 percent of patients. (14). In the diabetic foot, it is well recognized that suppurative and neuropathic changes commonly coexist. The major challenge in diagnoses then becomes distinguishing between soft-tissue infection from bone infection and infectious from non-infectious neuropathic bone lesions. Because many diabetic patients thought to have osteomyelitis actually have Charcot changes, this differentiation becomes crucial in deciding which patients require antimicrobial therapy.
Diagnosing any patient that is suspected of having osteomyelitis starts with a history and physical examination of the lower extremity. Pertinent historical findings would include a medical history denoting any conditions that would predispose a patient to neuropathy, peripheral vascular disease, decreased wound healing ability and compromised immunity. Certainly diabetes mellitus would be an important finding, but there are other conditions that may complicate the Medical picture, for instance other endocrinopathies and other foot problems that can lead to neuropathy and vasculopathy. Patients who have had an ulceration, especially over a bony preeminence, for over two weeks are at risk for contiguous bone infection. Previous osteomyelitis is a risk factor for a re-occurrence of bone infection.
Routes of infection
There are four main routes that bone normally becomes infected.(3) 1) Organisms infect the bone through the blood stream. This is called hematogenous osteomyelitis and the susceptibility of any bone is dependent on the patency of the surrounding blood flow. In this case, involvement of the marrow and medullary bone is early with later involvement of the cortex and periosteum. 2) Spread of infection from a contiguous source is the most common mechanism of infection in the adult patient. In this case the direction of infection is opposite that of hematogenous osteomyelitis in that the infection spreads from the periosteum and cortex to the medullary bone and marrow. 3) An important cause of bone infection is direct contamination of the bone with infective material through puncture wounds and penetrating injuries. 4) A major contributing event to osteomyelitis is post-operative infection. This mode of infection is multifactorial and can incorporate spread to the bone from a contiguous septic focus, direct implantation, and/or hematogenously.
Osteomyelitis usually, but not always, presents with local symptoms such as purulent drainage, erythema, edema, and sometimes pain. Fever is not common unless the patient is bacteremic, which is in itself unusual. Other clinical findings that are helpful in the diagnosis are peripheral neuropathy and loss of protective sensation. The usual cause of the diabetic foot osteomyelitis stems from an infection of the soft tissues of a larger ulceration, referring to its size and depth. It was found by Newman and colleagues that the larger and deeper the skin ulceration is, the more likely there is underlying osteomyelitis. An ulceration larger than 2 cm2 and deeper than 3mm were found to be predictive of underlying osteomyelitis (15). In addition, in one study, 89 percent of all infected ulcerations where a bony surface was contacted after using a sterile blunt probe were found to involve bone infection (16). However this same study points out that not probing to bone cannot exclude osteomyelitis.

The erythrocyte sedimentation test (ESR) is a useful tool in diagnosing osteomyelitis. The incidence of bone infection in patients with foot ulcers increases as the ESR increases. Lipsky and colleagues (3) found that an ESR over 40 mm/h was associated with nearly a 12-fold increase in likelihood of osteomyelitis in patients with suspicious ulcerations. Newman et al. Found that 100 percent of patients with ulcerations who had an ESR of over 70 mm/h had osteomyelitis. (15) A sensitive method for early diagnosis of acute staphylococcal osteomyelitis is the detection and quantification of teichoic acid antibodies. (8) This is a valuable way of assessing the clinical response as well as determining antibody levels in patients who are on antibiotics but who have a continuous foci of infection. While the WBC count has questionable effectiveness in diagnosing osteomyelitis,(3), the commonly employed laboratory tests used in diagnosing and monitoring the treatment of osteomyelitis include a CBC with differential and chemistry panel.
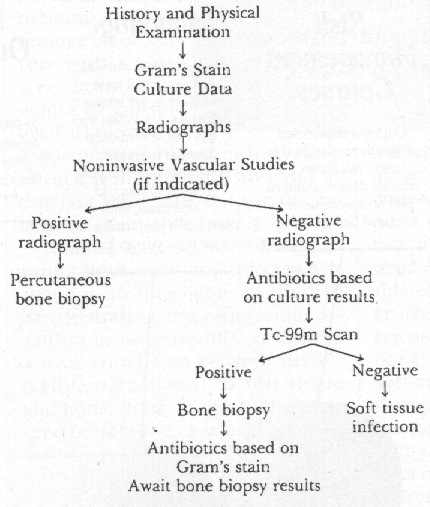
The definitive diagnosis and gold standard in diagnosing osteomyelitis of any type remains bone biopsy. A specimen should also be sent for aerobic, anaerobic, acid fast, and fungal culture and sensitivity. This then will confirm that osteomyelitis is present and indicate the offending pathogen(s). In patients who have negative cultures as a result of being on antibiotic therapy, a bone sample will provide material for a tissue gram stain. The bone biopsy will allow a histopathological diagnosis of osteomyelitis, which will demonstrate necrosis, infiltration with leucocytes or chronic inflammatory cells (lymphocytes or plasma cells). There may be other histological features of inflammation as well. In the case of probable hematogenous osteomyelitis, a positive blood culture with a positive bone scan would be considered diagnostic. Two to three blood cultures are needed from different sites and at different times. The test is more accurate if done while fever is spiking.
Studies have shown (15) that culturing sinus tracts and infected soft tissue is an unreliable method of demonstrating the responsible organism in osteomyelitis, as there is a weak correlation between the infective organism(s) of soft tissue and bone. Bone culture is the most specific method, but one must be aware of the inherent risks and potential complications of this invasive procedure. The risks, such as inadvertent bacterial seeding, are accentuated in the anatomically complex foot and situations may arise where bone biopsy may be difficult to obtain. In addition, many surgeons feel that the trauma of this invasive procedure in patients with vascular compromise may incite bone necrosis and infection. (17) In the proper candidate, if a suitable non-infected route to obtain the bone culture is obtained, a bone biopsy and culture would be the ideal approach.
If a patient has clinical and laboratory evidence of osteomyelitis, but a negative bone culture is obtained, the diagnosis can not be ruled out. In the face of cost containment, it is advised (18,19) a bone culture be considered mostly when the diagnosis or likely pathogen is in doubt. Otherwise, investigators (18) suggest that bone debridement followed by a soft-tissue guided oral antibiotic therapy for 10 weeks will result in the same desired outcome. Some investigators (3) state that all patients with osteomyelitis need to be hospitalized initially and receive intravenous antibiotic therapy.
Imaging modalities useful in the diagnosis of osteomyelitis
Knowing the pathological dynamics of a bony infection is important in the evaluation of osteomyelitis with various imaging techniques. There is a period of at least ten days between the onset of bony infection and any evidence of bony changes. However, soft tissue changes may be seen after approximately three days. The causative bacteria collect in the capillaries of the metaphysis and the epiphysis of the recipient bone causing abscesses and bony destruction. The infection may spread to the articular cartilage of the joint or invade the periosteum, the cortex and the medullary canal. Most commonly pus spreads to the subperiosteal space stripping it from the cortex and rupturing periosteal blood vessels. Nutrient blood vessels, the other source of blood supply to the bone, are occluded by bacterial emboli. Deprived of its circulation, the bone dies. The periosteum, still maintaining its osteoblastic ability forms new bone, called involucrum, around the dead bone, called sequestrum.
New bone formation also takes place in the cortex and the endosteum. Osteoporosis develops in the surrounding normal bone from disuse and/or the action of toxins. Cloaca, which are defects in the involucrum, develop and allow the discharge of pus and debris into the surrounding area. If the bone goes on to healing, the involucrum remodels and assumes a normal contour. If the infection becomes chronic the process may continue indefinitely or may become a localized abscess or sclerosing osteitis.
Certain imaging studies may be useful in the evaluation of osteomyelitis. These are: 1) Conventional Plane Film Radiography 2) Multiplanar Tomography 3) Computed Tomography 4) Radionuclide Bone Scanning 5) Magnetic Resonance Imaging. (8)
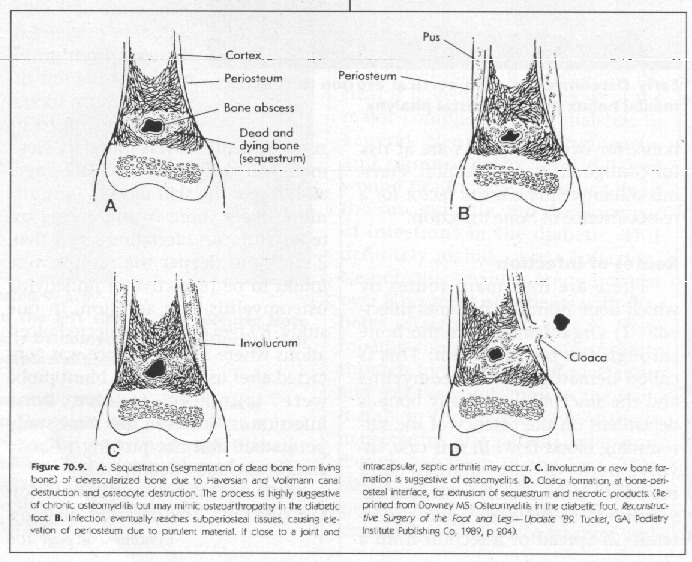
X-ray changes related to osteomyelitis are generally not visible until 10-20 days after infection when 30-70 percent of the bone has been absorbed. These changes are due to inflammatory hyperemia and secondary deossification and osteopenia in the acute phase. Focal osteopenia usually shows up as lucencies in the cortex or medullary bone. Sequestrum, involucrum and cloaca formation develops as the infection becomes chronic. These X-ray changes are not pathognomonic for osteomyelitis, but a clinical diagnosis can be made if these classic changes are accompanied by clinical findings, such as an ulceration that probes to bone. Periosteal new bone formation appears as a solid, cloaking pattern, forming the involucrum. The bony cortex becomes necrotic and sequestered, showing up as defects in the bone.
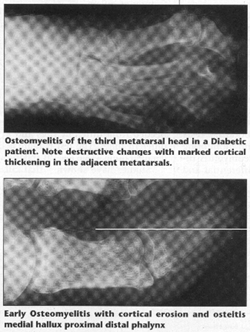
Regional osteoporosis occurs and the sequestrum, separated by granulation tissue, shows up as a chalky white radiodensity. Sequestrum vary in size and may be inside of the involucrum or makes its way outside of the involucrum through defects called cloaca. This leaves areas of intact cortex interspersed with necrotic segments. This distinguishes osteomyelitis from a malignant tumor, whereas malignancies destroy the cortex uniformly. Antibiotic treatment slows down the destructive phase and the formation of sequestrum and therefore accentuates the new bone involucrum formation seen. In the radiologic exam of the Diabetic foot, it is important to distinguish non-infectious destructive lesions from osteomyelitis.
The most common cause of these non-infectious lesions in a diabetic patient is a condition known as Charcot deformity or neuropathic osteoarthropathy, the foot being the site of greatest predilection. Because of severe neuropathy, the bones usually in the tarsal area fracture and then undergo bone destruction, resorption, sclerosis and new bone formation. The radiographic picture can mimic osteomyelitis. Some experts (13) say that if there if an opening in the skin overlying the area where these bony changes are present, then the clinician is compelled to treat this lesion as osteomyelitis. However, some studies have shown that about half of all lesions suspected of osteomyelitis were in fact Charcot fractures.(20,21)
This imaging technique shows internal detail of bone with high resolution and is used sometimes to detect smaller sequestra.
Computed Tomography
This imaging modality is useful in that in some patients where there are no findings of osteomyelitis on plane films, CT reveals positive findings. CT scanning is more sensitive to cortical bone involvement and is better at detecting sequestration in chronic or partially treated osteomyelitis. In addition, increase in the density of the marrow cavity due to the inflammatory response can be detected in early osteomyelitis with CT as well as the presence of pus, especially when associated with bony erosion. (8) This modality shows cross-sectional anatomical detail with good spatial resolution. However, the contrast resolution and sensitivity are lower than with MRI.
Radionuclide Bone Scanning
This modality is very useful in the investigation of bone infection, as it reflects osteoblastic activity as well as skeletal vascularity. Therefore, the bone scan can become positive 24 hours after the onset of the infection. The importance of the bone scan lies in its role for early detection, differentiation of osteomyelitis from cellulitis, and identification of renewed activity in some cases of chronic osteomyelitis. An important limitation of radionuclide bone scanning is that it is not specific for osteomyelitis. Any preexisting osseous condition that causes bone turnover, like healing fractures, previous surgeries and neuropathic osteoarthropathy will likely show a positive bone scan. Therefore, bone scanning is valuable only when the suspected osteomyelitis is not superimposed on another disease that causes bone remodeling.
Technetium 99mdp is the current radioactive material of choice. This is a highly accurate exam and maintains its accuracy through any duration of symptoms, skeletal sites and age groups. The uptake of this isotope is dependent on an intact blood supply, therefore will not accumulate in infected bone around which there has been thrombosis of arteries and capillaries. It is commonly employed as a three- or four-phase study, which improves specificity. The initial phase (flow), recorded immediately, displays relative perfusion and thus demonstrates areas of decreased flow. The second phase (blood pool) is recorded within 5- 10 minutes and best reveals soft tissue involvement. The third phase (delayed) is recorded 2-4 hours later and the fourth phase is read at about 24 hours after the initial injection of the isotope. Urinary excretion removes most of the isotope by hour two thus removing the soft-tissue background, and will enhance the osseus uptake of the nuclear material. By comparing the delayed phase with the prior studies, cellulitis can be differentiated from osteomyelitis.
If the Technetium 99mdp bone scan is normal in patients with suspected osteomyelitis, a Gallium-67 scan is sometimes used to further differentiate soft tissue vs. bone infection, as the isotope becomes concentrated at the site of inflammation. Gallium- 67 citrate interacts with iron binding sites on serum proteins transferrin, lactoferrin and ferritin as well as white blood cells and bacteria. Gallium scans are usually reserved for soft tissue infection because of these properties.
Indium 111 interacts with white blood cells. First, the white blood cells must be harvested and labeled with indium. This is then re-injected into the patient. It is a time consuming test, requires expertise and is very expensive. However, studies comparing this test to other imaging modalities in diagnosing diabetic foot osteomyelitis suggest it is the most accurate of the radionuclide studies (3) . It is the first modality to be able to differentiate Charcot fractures from acute osteomyelitis. In chronic osteomyelitis, because the physiologic response is primarily lymphocytic, the scan eventually becomes negative. Because of the scan normalizing as the infection subsides, this test can potentially be useful in following the response to antibiotics. The major limitation of this study is that poor resolution may preclude differentiation of soft tissue and bone infection. Combining this study with a Technetium 99mdp study or 99mTc-labeled sulfur colloid study, which concentrates in the marrow, can improve specificity.
Tc-99m HMPAO labeled leucocytes offers a more cost-effective alternative to indium. Less toxic, it therefore allows for a larger dose for better resolution. Images can be collected within 3-4 hours allowing a more prompt and accurate therapeutic intervention.
Both Indium 111 and Tc-99m HMPAO are effective at differentiating acute osteomyelitis from other inflammatory and/or bony processes, but distinguishing an inflammatory response from a low grade early bone infection or delineating some types of chronic osteomyelitis remains a clinical challenge. (8)
MRI
Magnetic resonance imaging may offer the most promising future of all the imaging techniques. In recent studies of patients with foot infections and suspected osteomyelitis, (3) MRI was more effective in detecting infection of the bone than plain radiography or bone scans. In addition, MRI, in most cases, can differentiate between soft tissue infection and extension of the process to involve bone. Changes within the periosteum and bony cortex can also be detected with a high degree of confidence. Initial involvement of bone from a contiguous soft tissue infection can be seen on high-resolution images using both T1-weighted and T2- weighted or STIR sequences. (14) . As osteomyelitis becomes chronic, the cortical changes are typically more extensive than marrow changes, and the remodeling of the cortex and the medullary cavity of long bones is well demonstrated using MRI. This differentiation of acute from chronic osteomyelitis and the ability to distinguish any suppurative bone involvement from neuropathic changes that take place in diabetic neuroarthropathy, is a result of its high tissue contrast, increased spatial resolution and increased sensitivity to medullary changes not feasible with other modalities, as well as the ability of the MRI to differentiate between the layers of the foot and soft tissue from bony involvement.
Distinguishing healed osteomyelitis from surrounding active disease, however, may pose a challenge using MRI. There are clinicians, though, that are able to make this distinction on the basis of an increased marrow signal on T1-weighted images. (14).
Bacteriology
Most cases of osteomyelitis in the diabetic foot usually are a result of infectious spread from contiguous soft-tissue or develop as a result of a post-operative infection. (22) The most common pathogen found is S. aureus (3). Other common pathogens found are S. Epidermis and Streptococci. These three organisms account for 73 percent of osteomyelitis in the diabetic foot. Recent studies suggest that enterococci and gram negative bacilli are not an uncommon source of infection. (17) This is presumably due to the over-use of prescription antibiotics. When a bone infection follows foot implant surgery, S. Epidermis is usually the predominate organism. Some infectious disease specialists suggest prophylactic antibiotics for implant patients due to the ability of gram negative organism's ability to adhere to the implant and produce a slime glycolax layer.(13) Infections due to Pseudomonas Aeroginosa represent less that 10 percent of all diabetic foot osteomyelitis cases (23). Kalish and Mahan reported it to be the most common pathogen in puncture wounds to the foot, especially of the calcaneus, and in particular those that were wearing rubber- soled shoes. (23) Pseudomonas is also a common pathogen in patients who have been soaking their foot.
Polymicrobial infections, common in puncture wounds in general and in osteomyelitis, contain fewer bacterial species in bone infection than in soft-tissue infections. Gram negative or mixed infections are said to be more common in the compromised host (i.e. peripheral vascular disease, diabetes mellitus) (23). In addition, anaerobic infections are on the rise in diabetic infections. (23) Diabetes is a frequent condition found among patients with anaerobic osteomyelitis. In general, however, anaerobic bacterial contaminants are more common in soft tissue infections than in diabetic osteomyelitis, but the more severe the infection, the higher the likelihood of finding anaerobic pathogens. (3) In addition, anaerobes are more frequent in infections that are long- standing, accompanied by necrotic tissue, have a foul odor and have failed previous antibiotic therapy. One study (13) found that 30 percent of diabetic osteomyelitis contain anaerobic pathogens, with bacillus fragilis being the most common. Salmonella has been associated with osteomyelitis in sickle cell disease as being the predominate organism. If anaerobic cultures are not available but there is a high index of suspicion, antibiotic therapy directed toward anaerobes is warranted.
Microorganisms, such as staphlococcus epidermis, corynebacterium and coagulase negative staphlococcus, normally considered to be harmless contaminants in other settings, have been found (3) to be pathogenic in diabetic foot osteomyelitis. In addition, it has been well-documented (3) that the pathogens found in the soft tissue is a poor predictor of the organisms found in bone cultures. Cultures should be taken with the precautions, and should separately contain specimens of infected bone and the overlying soft tissue infection to increase the likelihood of isolating all pathogenic organisms.
Treatment
Optimal treatment of the diabetic patient with osteomyelitis of the foot requires the skillful interplay of different disciplines, including a primary care physician, specialists in radiology, microbiology and infectious disease, wound management, vascular evaluation and surgery as well as foot surgery. After establishing the diagnosis of diabetic foot osteomyelitis, and before a treatment program is instituted, the following information must be obtained: 1. The medical status of the patient (febrile, allergies, control of Diabetes) 2. The site and extent of the bone and soft tissue infection 3. The vascular status of the patient 4. Results of culture and sensitivities, and bone biopsy 5. Determination of the presence of swelling, gangrene, necrosis, evidence of tissue gas, and whether there is a rapidly progressive infection.
The medical status of the patient should be evaluated especially in regarding allergies to antibiotics and control of the diabetes. Bacteremia and fever are not common findings with diabetic foot osteomyelitis, but if present, this must be a priority consideration. A determination of the exact site and extent of infection should be should be made on the basis of clinical exam and imaging studies. One cannot assume that the soft tissue infection contains the same pathogens as the bone infection and both must be considered as separate entities. Knowing which bones on the foot are infected allows proper determination of the extent of debridement and/or amputation and is important information that will allow the surgeon to create a functional postoperative foot such that the patient will ambulate maximally after any ablative procedure.
For example, the usual sites of diabetic foot osteomyelitis (17) were the metatarsal bones and the proximal phalynx. If the infection is limited to these sites, a transmetatarsal amputation will still allow the patient a surface on which to ambulate. Vascular studies such as Doppler exams, blood pressures of the foot, photoplethysmography, and TCPO2's are essential in determining whether antibiotics can penetrate the infected area and whether any surgical debridements and/or local amputations will be successful. Medical treatment of foot osteomyelitis in the diabetic patient with severe vascular insufficiency is usually futile and limb amputation is almost inevitable. (17) Systemic hyperbaric therapy has been shown to potentiate antibiotics, increase circulation and thereby assist the vascularly compromised patient.
The results of culture and sensitivities and bone biopsy will help confirm the clinical diagnosis and guide the antibiotic treatment. If the patient has evidence of an abscess, necrosis and/or gangrene, tissue gas, or a rapidly progressive infection, then immediate surgical intervention is necessary. Research done at a major university showed that the absence of these conditions, the presence of swelling and the use of high dose antibiotics directed against the identified pathogens for at least four weeks intravenously, or combined with oral antibiotics for ten weeks, predicts a positive outcome (17).
A common viewpoint held is that conservative medical and surgical treatment is rarely successful and amputation is the treatment of choice. (3) This viewpoint was disputed by researchers that showed the value of conservative medical and local debridement.(17) The reasons for previous failure of conservative treatment is said to be a result of improper choice of antibiotics, severe arteriosclerotic peripheral vascular disease and delayed attempts at revascularization, unreliable cultures, lack of aggressive early high dose systemic antibiotics, delayed and unsubstantial debridement of dead bone and tissue. In addition, many successful patients stay on oral antibiotics until the foot ulcer is healed and there is no clinical or radiologic evidence of pathology at the site in question.
It is clear then that diabetic foot osteomyelitis has a good chance at healing when treatment is guided by the clinical signs of potential positive outcome, (i.e., adequate vascular status of the patient, presence of swelling), the necessity for local debridement, proper local surgical intervention and choice of procedures, proper choice of antibiotics, combinations used and the method of administration and their aggressive and early use.
In summary, caring for the podiatric patient who is diabetic requires uncompromising precision in identifying risk factors for the development of foot ulceration and infection, implementing prevention models, developing diagnostic skills, and ability to offer state of the art treatment modalities. This requires working as a vital link in an interdisciplinary team and knowing when and who to refer to specialists. This will enable the podiatrist to do what no professional can do better: care for the diabetic foot and be the team leader for lower extremity amputation prevention.
References:
1. Avioli LV: Osteomyelitis. In: Wyngaarden JB, Smith LH, eds. Cecil Textbook of Medicine 16th ed. Philadelphia: WB Saunders, 1982; 1349-1350.
2. Little JR, Kobayashi GS, Sonnenwirth AC: Infection of the diabetic foot. In: Levin ME, O Neal LW, Eds. The Diabetic Foot. St Louis: CV Mosby, 1983; 133-147.
3. Lipsky BA; Osteomylitits of the Foot in the Diabetic Patients, Clinical Infectious Diseases, 1997; 25: 1318-1326.
4. Gibbons GW, Eliopoulus GM: Infection of the diabetic foot. In: Kozak GP Hoar BS, Rowbotharn JL, Wheelock FC Gibbone GW, Campbell D, eds. Management of diabetic foot problems. Philadelphia: WB Saunders, 1984; 97-102.
5. Friedman SA, Rakow RB: Osseous Lesions of the foot in diabetic neuropathy. Diabetes 1971; 20: 302-307.
6. Connell FA. Shaw C. Will J. Lower extremity amputation among persons with diabetes mellitus- 1980-1987. MMWR Morb Mortal Wkly Rep 1991; 40:737-9.
7. Geiss LS, Herman W, Goldschmid MG, et al. Surveillance for diabetes mellitus 1980-1987. MMWR Morb Mortal Wkly Rep 1993; 42:1-20.
8. Greenfield GB, Osteomyelitis, Radiology of Bone Diseases, 1990:474-508.
9. McGlamry, ED Banks AS Downey MS. Comprehensive Textbook of Surgery, 2nd edition, Williams And Wilkins, 1992:1719-1743.
10. Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clincial features therapeutic considerations and unusual aspects. N Engl J Med 1970;282:316-7.
11. Cierny G, Mader JT, Pennick JJ. A clincial staging system of adult osteomyelitis. Contemporary Orthopaedics 1985;10:17-37.
12. Caputo GM, Cavangh PR, Ulbrecht JS, Gibbons GW, Karchmer AW. Assessment and management of foot disease in patients with diabetes. N Engl J Med 1994;331:854-60.
13. Lecture by Matthews R, Care of the Insensitive Foot. The Carville Approach. October 6-8, 1993.
14. Deutsch AL, Mink JH, Kerr R, Bone Infection: MRI of the Foot and Ankle 1992;205-214.
15. Newman LG, Waller J, Palestro CJ, et al. Unsuspected osteomyelitis in diabetic foot ulcers: Diagnosing and monitoring by leikocyte scanning with indium in oxyquinolone. JAMA 1991 266:1246-51.
16. Grayson ML, Gibbons GW, Balogh K, Levin E. Karchmer AW. Probing to bone in infected Pedal ulcers. JAMA 1995;27:721-3.
17. Bamberger DM, Daus GP, Gerding DN, Osteomyeltis in the Feet of Diabetic Patients, The American Journal of Medicine 1987, Volume 89;653-660.
18. Eckman MH, Greenfield S. Mackey WC, et al. Foot infections in diabetic patients: decision and Cost-effectiveness analysis. JAMA 1995;273: 713-20.
19. Mushlin A, Littenburg B. Diagnosing pedal osteomyelitits: testing choices and their consequences. J Gen Intern Med 1994;9:1-7.
20. Lipsky BA. Pecoraro RE. Harley JD. Et al. Diagnosis of bone lesions in the diabetic feet: Osteomyelitis or osteoarthroapthy? In: Program and abstracts of the 29th Interscience Conference on Antimicrobial Agents and Chemotherapy ( Houston, TX). Washington. DC: American Society for Microbiology. 1989.
21. Lipsky BA, Pecoraro RE, Harley JD. Et. al. Foot bone lesions in diabetic patients: diagnosis and natural history. Diabetes 1991;40(suppl):553A.
22. Conference-Super Seminar, Detroit, MI May 9-12-1995; Diabetic Podiatric Care, Lecture by Jenkin, WM.
23. Conference-Diabetic Foot and Wound Care; Orlando, FL November 13-16 1997; Lecture by Robbins, WJ.